Lesedauer von 4 Min.
09. April 2025
ATEMWEGSGESUNDHEIT
Artikel
Real-world clinical impact of in-house PCR testing
In a post-pandemic world, the importance of rapid, reliable, and comprehensive diagnostic testing for respiratory illnesses has never been clearer. A recent study1 comparing the use of multiplex point-of-care (POC) molecular testing with laboratory-based molecular testing for influenza-like illness (ILI) in the United States provides compelling support for a shift in clinical practice to meet this need.
Real-world comparison of POC vs laboratory-based molecular testing
The study team compared two groups of patients presenting with ILI symptoms: those who received point-of-care testing using Xpert® Xpress CoV-2/Flu/RSV plus (Xpert Xpress) tests and those who underwent other laboratory-based molecular testing. The study assessed outcomes on the day of patients' initial visit and up to 90 days of follow-up from January 2021 to September 2022 and represents a real-world analysis of the use of tests for SARS-CoV-2 and their impact on patient outcomes.
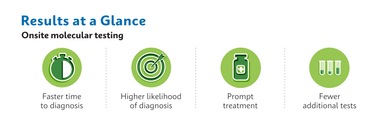
Compared to send-out molecular testing, outpatients who were tested with Xpert Xpress were diagnosed more quickly for COVID-19, flu, and RSV (zero vs four or more days) and were more likely to be treated (7,4% vs 4,3%). Additionally, the Xpert Xpress group received treatment more quickly for diagnosed infections compared to laboratory send-outs (one vs five days). Patients tested with Xpert Xpress had less additional testing after the initial visit date (<15% of patients had two or more tests, vs 50% for the lab send-out group) and were more likely to be diagnosed with COVID-19, flu, and RSV, highlighting improved diagnostic accuracy. Taken together, these results indicate a potential for lower resource utilization for testing with the Xpert Xpress POC approach.
Nearly one-third of Xpert Xpress testing occurred in emergency departments, highlighting the utility of molecular POC testing in acute care settings, in line with recent findings that implementing POC PCR improves clinical metrics and healthcare resource utilization.2–4
Study co-author, Jordan Chase noted, “This study highlights the clinical benefits of on-site patient testing with Xpert Xpress and underscores the role of cutting-edge diagnostic solutions in advancing patient care.”
The findings from this study provide evidence to support the broader adoption of point-of-care molecular testing in clinical practice.
Advocating for molecular testing in outpatient sites
Healthcare workers and administrators play a pivotal role in the transition towards more efficient diagnostic practices. Building a case for the initial investment requires an analysis of the potential clinical, financial, and operational implications.
Here are some key considerations in making the case for investing in onsite molecular testing:
- Patient satisfaction metrics
- Accuracy and timeliness of results
- Current number of call-backs and patients lost to follow-up
- Current indirect costs e.g. antiviral/antibiotic usage, patient isolation, and repeat/additional testing
- Potential for diagnostic stewardship (i.e., prioritizing onsite molecular tests for appropriate patients)
- Community health and the impact of reducing transmission
- Ease of use and incorporation into clinical workflows, including integration into electronic health records
- Potential to support multiple test types on a single testing platform
- Manufacturer support in setup and training
- Reimbursement landscape
Conclusion
The evidence is clear: point-of-care molecular testing offers significant benefits that align with modern healthcare goals. Healthcare workers can leverage technology such as Xpert Xpress testing to enhance patient care, improve operational efficiency, and enable better outcomes.
See the real-world evidence here
IVD. In Vitro Diagnostic Medical Device. Bestimmte Tests sind eventuell nicht in allen Ländern erhältlich.
Literatur
1. Stockl KM, Tucker J, Beaubrun A, Certa JM, Becker L, Chase JG. Real-world use of multiplex point-of-care molecular testing or laboratory-based molecular testing for influenza-like illness in a 2021 to 2022 US outpatient sample. PLoS ONE. 2024;19(11):e0313660. doi:10:1371/journal.pone.0313660
2. Hinson JS, Rothman RE, Carroll K, et al. Targeted rapid testing for SARS-CoV-2 in the emergency department is associated with large reductions in uninfected patient exposure time. J Hosp Infect. 2021;107:35-39. doi:10:1016/j.jhin.2020:09.035
3. Davies S, Boller E, Chase J, Beaubrun A, Miller C, Jensen I. A cost-consequence analysis of the Xpert Xpress CoV-2/Flu/RSV plus test strategy for the diagnosis of influenza-like illnesses. J Med Econ. 2024;27(1):430-441. doi:10:1080/13696998:2024:2313391
4. Fenstermacher K, Klein E, Mumford J, et al. Pre- and Post-implementation Comparison of the Impact of Emergency Department-Based COVID-19 Point-of-Care Testing on Emergency Department Patient Metrics. Annals of Emergency Medicine. Oktober 2023. Zugriff am 15. Januar 2025. https://www.annemergmed.com/article/S0196-0644(23)01079-X/fulltext
Nächsten Artikel lesen
MEHR








